Zantac
- Zantac Litigation
- Zantac Legal History
- Zantac Litigation Status & Recent Favorable Rulings
- Potential Zantac Settlement and Compensatory Value
- Potential Injuries and Legal Claims Arising from Zantac Lawsuits
- How can I find qualified Zantac leads?
- TV Commercials
- Infomercials
- Landing Pages
- Social
- Paid Search
Zantac Litigation
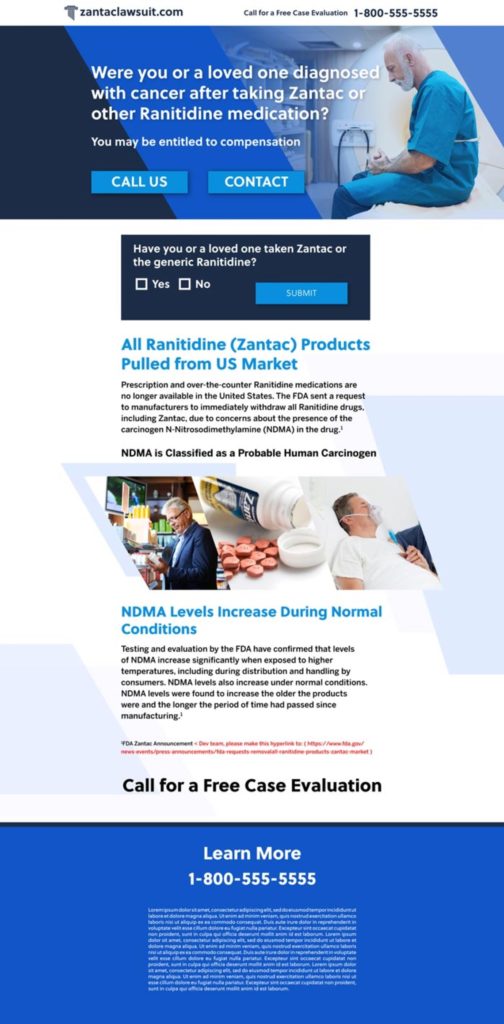
Zantac is the brand name for the antihistamine and antacid ranitidine, an H2 (histamine 2) blocker. Pharmacies and stores sell ranitidine over the counter and by prescription as medication to prevent and relieve heartburn. However, Ranitidine medicines, including Zantac, contain a nitrosamine impurity called N-nitrosodimethylamine (NDMA). The Environmental Protection Agency (EPA) has classified NDMA as a “B2 probable human” carcinogen, which means NDMA can cause cancer in animals, but the EPA has not yet definitively determined it to cause cancer in humans.
Scientists have determined that, over time, NDMA levels increase in ranitidine. These levels also increase more rapidly when ranitidine is stored at higher temperatures. Experts have linked NDMA to several types of cancers affecting the following bodily organs and parts:
- Stomach
- Liver
- Colon
- Prostate
- Breasts
- Kidneys
- Thyroid
- Bladder
In 2019, a company tested a batch of Zantac and detected NDMA in the product. The detection of NDMA in Zantac has led to an FDA warning, widespread product recalls, and thousands of lawsuits.
CAMG is the nation’s leading consumer attorney marking firm, with comprehensive services designed to provide law firms with all the tools they need to target potential plaintiffs in mass tort litigation. Our creative and marketing teams at CAMG can help you find the best-qualified leads for your firm’s Zantac litigation.
Zantac Legal History
In September 2019, online pharmacy Valisure notified the U.S. Food and Drug Administration (FDA) that it detected NDMA in some Zantac batches. That same month, the FDA issued a statement “alerting patients and health care professionals of NDMA found in samples of ranitidine.” The FDA announced that it was studying whether low levels of NDMA pose a risk to consumers. In April 2020, the FDA issued a press release requesting manufacturers to “withdraw all prescription and over-the-counter (OTC) [Zantac] from the market immediately.”
After the FDA issued its statement, numerous companies issued voluntary recalls of over-the-counter ranitidine medicines. Walgreens, Walmart, CVS, and Rite Aid pulled Zantac (both over-the-counter and prescription) off the shelves. Fourteen generic manufacturers and Zantac’s brand-name manufacturer Sanofi-Aventis recalled Zantac in the United States.
Zantac consumers who developed cancer filed thousands of lawsuits against manufacturers of Zantac and other ranitidine medications. Manufacturers named in lawsuits include Pfizer, Sanofi-Aventis, and Boehringer Ingelheim, with claimants alleging Zantac or ranitidine caused their cancer. The lawsuits assert that the manufacturers knew that ranitidine contains a known carcinogen but failed to warn consumers. Consumers have also sued generic manufacturers and retail stores that sold the heartburn medicines.
The many lawsuits have been consolidated in multidistrict litigation (MDL) currently pending in the Southern District of Florida.
Zantac Litigation Status & Recent Favorable Rulings
As of September 2021, hundreds of lawsuits (700+) are pending in the MDL against brand name manufacturers of Zantac as well as against generic manufacturers and retailers, alleging false advertising, failure to warn, and negligence claims, and requests for medical monitoring for people who have not yet contracted cancer.
The MDL, In Re: Zantac (Ranitidine) Products Liability Litigation, was established in February 2020. In July 2021, U.S. District Judge Robin Rosenberg dismissed the claims in the MDL Master Complaint against generic manufacturers of Zantac and retailers that sold Zantac but allowed suits to go forward against name-brand manufacturers, including Pfizer, GlaxoSmithKline, Boehringer Ingelheim, and Sanofi-Aventis.
As for the dismissed claims in the MDL Master Complaint, the U.S. District Court noted that individual plaintiffs could still initiate separate claims against the retailers if they could show that a specific one was negligent. For example, a plaintiff could indicate that the retailer stored ranitidine at unsafe temperatures, causing high levels of NDMA.
The Court also dismissed the medical monitoring claims and federal RICO claims. However, it allowed many other claims alleged in numerous lawsuits to go forward, including claims for negligence, wrongful death, design defect, and punitive damages. The Court also allowed some plaintiffs to amend their complaints to seek medical monitoring under various state laws.
More than 1,400 lawsuits are pending in the MDL, and more than 70,000 more claims have been registered with the court.
Potential Zantac Settlement and Compensatory Value
As litigation moves forward against Zantac manufacturers, they’ll likely want to settle lawsuits rather than litigate the claims. The sheer number of current and potential plaintiffs means litigation will be extremely costly. By settling claims, Zantac manufacturers will save millions of dollars.
The compensatory value of each Zantac claim can vary widely. For some plaintiffs, it’ll be only the purchase price of Zantac. In contrast, plaintiffs who have contracted cancer may be able to obtain significant settlements, including punitive damages, for their major, life-altering, or deadly injuries.
Potential Injuries and Legal Claims Arising from Zantac Lawsuits
Plaintiffs who don’t have cancer but suffered economic losses by purchasing Zantac may still initiate claims. These claims can include:
- Failure to warn
- False advertising
- Breach of implied and express warranty
- Claims for medical monitoring in certain states
Plaintiffs who have cancer after using Zantac could bring claims for design defect, failure to warn, negligence, and even wrongful death.
All plaintiffs can file claims against the name-brand manufacturers of Zantac, but generally not against generic manufacturers and retailers. A plaintiff could potentially bring a claim against a retailer that sold Zantac if that plaintiff can show the retailer was negligent in storing the heartburn medication, thereby increasing its likelihood of containing high NDMA levels and being essentially toxic to consumers.
How can I find qualified Zantac leads?
Our team at CAMG works solely for law firms, and we have proven success in strategically targeting clients for mass tort litigation. We can help you find qualified leads in Zantac litigation by optimizing your search engine results and targeting key clients methodically across all media forms. We also provide numerous support services, including 24/7 intake call systems, medical records retrieval, and a streamlined process for securing contracts with clients. We offer bundled and a la carte services, and we will work with you to make sure the assistance we provide is specifically tailored to your firm’s unique needs.
Contact us now to find out how we can help your firm get started with Zantac litigation.
TV Commercials
Infomercials

Landing Pages
Social



Paid Search
CAMG has thousands of spots and infomercials that can be branded for your firm. Reach out to us for the latest creative offline and online examples*.
*Examples shown are not always current examples.
Request Samples
* These fields are required.

Is your firm in the Top 3 of Google Search?

CAMG Ethics White Paper
Legal Areas
Are you looking for data-driven marketing for your law firm?
I have worked with CAMG for years. Not only are they great at delivering, but they are great people to work with!

CAMG is always accommodating and willing to work in a way that supports your office as an extension of your team.

Steve Nober and the team at CAMG are responsive, ethical, talented, reasonably priced, and easy to work with!

If you are looking for professional, hardworking people who produce great results and then take you out for a nice dinner, look no further than CAMG


THE NATION’S LARGEST FULLY INTEGRATED
Marketing Agency Dedicated to Law Firms
- Television
- Radio
- Public Relations
- Medical Record Retrieval & Review
- Search Engine Optimization
- Paid Digital
- Out of Home
- Intake & Contracting Services
