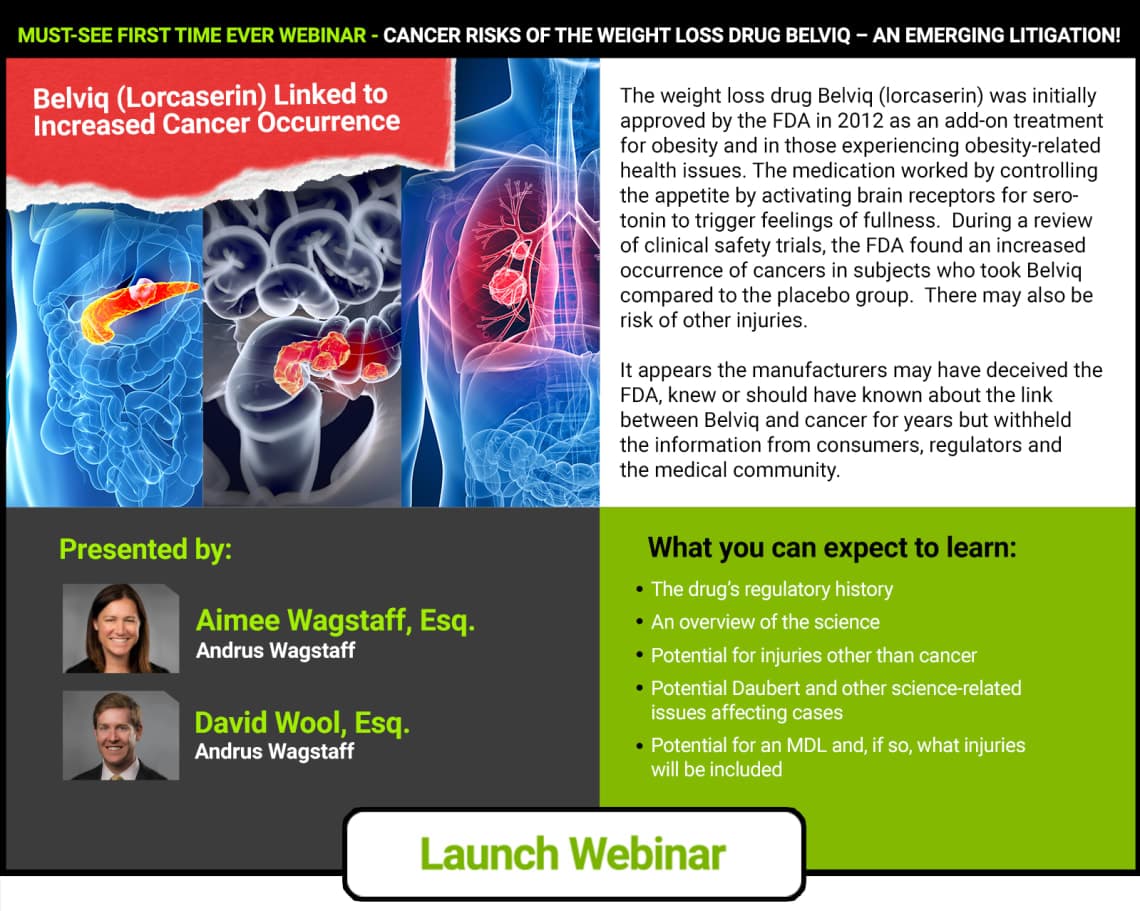
Cancer Risks Of The Weight Loss Drug Belviq – An Emerging Litigation
Navigate this page
Add a header to begin generating the table of contents
After agreeing to the below terms, you can watch the full webinar, download a copy of the powerpoint, and access documents pertaining to this case.
I hereby do depose and say under oath that I am not a member of the media. I do not work in any capacity for, nor have I been hired by, any law firm or in-house counsel defending any causes of action related to products liability, mass tort or otherwise nor am I employed in any capacity or hired by any company or corporation that manufactures, designs, sells, distributes or otherwise medical devices or pharmaceutical products. I understand that this webinar presentation was prepared in anticipation of litigation, is attorney work product and confidential, and that I will not use these materials or share what I learn from this presentation other than to prepare my own cases and/or to assist the prosecution. I understand that my attendance, participation or being shown any of the materials is conditioned upon my attestation under oath and agreement to the terms above.
Subscribed and sworn to under the pain and penalties of perjury.
***Typing your full name electronically shall serve as your signature.***

Is your firm in the Top 3 of Google Search?
Our comprehensive SEO program is designed to get you in the Top 3 Google Search results and keep you there. Contact us today for a complimentary audit and review.

CAMG Ethics White Paper
Now more than ever, ethics count. “A Lawyer’s Ethics Obligations When Participating in a Lead Generation Program,” authored by a team of ethics and compliance attorneys, is continuously updated with the latest rules.
Most Recent Webinars
Are you looking for data-driven marketing for your law firm?
As a full-service agency, CAMG handles everything from marketing and creative to the support your law firm needs to operate campaigns at the maximum efficiency.
What Our Clients Say


I have worked with CAMG for years. Not only are they great at delivering, but they are great people to work with!
I have worked with CAMG for years. Not only are they great at delivering, but they are great people to work with!

What Our Clients Say


CAMG is always accommodating and willing to work in a way that supports your office as an extension of your team.
CAMG is always accommodating and willing to work in a way that supports your office as an extension of your team.

What Our Clients Say


Steve Nober and the team at CAMG are responsive, ethical, talented, reasonably priced, and easy to work with!
Steve Nober and the team at CAMG are responsive, ethical, talented, reasonably priced, and easy to work with!

What Our Clients Say


If you are looking for professional, hardworking people who produce great results and then take you out for a nice dinner, look no further than CAMG
If you are looking for professional, hardworking people who produce great results and then take you out for a nice dinner, look no further than CAMG


THE NATION’S LARGEST FULLY INTEGRATED
Marketing Agency Dedicated to Law Firms
- Television
- Radio
- Public Relations
- Medical Record Retrieval & Review
- Search Engine Optimization
- Paid Digital
- Out of Home
- Intake & Contracting Services